From Pigs to People: The Future of Organ Transplants Is Here
Xenotransplantation is no longer science fiction. Gene-edited pig hearts and kidneys are offering new hope to patients and transforming transplant medicine.
By Tyler Santora
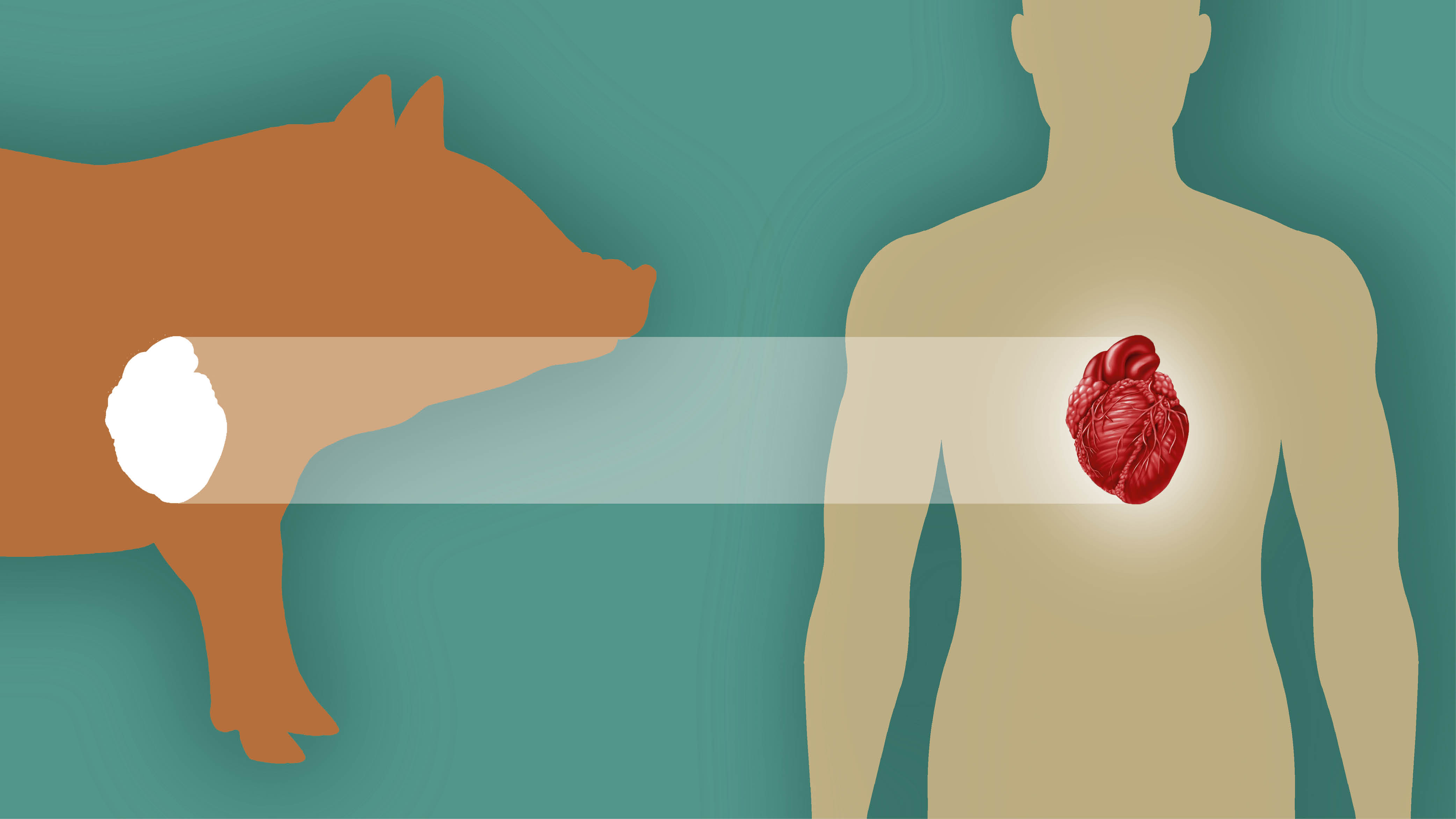
More than 103,000 people in the U.S. are waiting for a lifesaving organ transplant, and many of them will never have one. Every day, 17 Americans die while still on the waitlist. But a burgeoning field of medicine might be able to save these lives—and transform the field of transplantation in this country—by giving humans organs from animals, in a process called xenotransplantation.
“If we can provide this option, that will definitely meet the shortage of organs,” says Muhammad Mohiuddin, MBBS, director of the cardiac xenotransplantation program at the University of Maryland School of Medicine.
In some cases, these “xeno” organs could be used as a lifeline until a human organ is available, Mohiuddin says. But the goal is that this won’t be necessary, and the non-human organs would last for years, even decades.
Xenotransplantation has been around since at least 1682, when doctors replaced part of a Russian nobleman’s skull with a canine graft. Today, modern medicine is using gene-edited pig organs such as the heart, kidney and liver to transplant into people.
The modern study of xenotransplantation picked up in the late 1990s. Back then, Mohiuddin transplanted hamster hearts to rats and rat hearts to mice. After achieving success in small animal models, he moved to transplanting pig hearts to baboons.
At first, animal trials ended in organ rejection within minutes. But with recent advances in gene editing and immunosuppression, Mohiuddin and his contemporaries have completed pig-to-primate transplants over the past few years. Now, the field has turned to transplanting pig organs into humans. Pigs are good sources for organs because they grow fast and have large litters. They also have a well-studied genome and organs that are fairly compatible with humans.
The Challenges of Xenotransplantation
There are three aspects of xenotransplantation that make it challenging: rejection, infection and disease transmission.
Rejection is a challenge with all transplants, even within the same species. But it’s more complicated in xenotransplants because humans have antibodies that attack pig organs and their byproducts. Additionally, the techniques that allow doctors to catch rejection early in typical human-to-human transplants, allowing them to take life-saving measures, don’t work as well in xenotransplants, Mohiuddin says.
Physicians typically prevent rejection with immunosuppressive drugs, dulling the recipient’s immune system so it doesn’t attack the transplanted organ. For a long time, doctors thought the drugs they prescribe for typical transplant rejection wouldn’t work for xenotransplants. But United Therapeutics, a biotechnology company leading the xenotransplantation race, recently demonstrated that the medications used for human-to-human transplants work with a largely normal protocol in xeno recipients.
“We have learned a lot about these drugs … and we now have a way of monitoring them and giving just enough so we can prevent rejection,” Mohiuddin says. That’s crucial because there’s a delicate balance between dampening the immune system enough so that it doesn’t attack the xeno organ and keeping it active enough to prevent infections.
Scientists can also use an additional approach with xeno organs that they can’t with human ones: genetic engineering. For example, researchers have knocked out three pig genes that create sugars people have antibodies to. This reduces the chance of acute organ rejection—and how much immunosuppression recipients need.
“We overcame the risk of acute rejection. The challenges are mostly now in the long term. How will the immune system react months after a transplant?” says Leonardo Riella, MD, PhD, medical director of kidney transplantation at Massachusetts General Hospital.
Researchers have also added human genes related to blood clotting and immune function to the pig genome. This is important because complement activation systems—in which proteins called complements mark pathogens for destruction by other immune cells—are incompatible between pigs and humans. “We realized that by introducing some human regulatory genes … the survival of these kidney transplants were significantly greater,” Riella says.
With further research, scientists may be able to pinpoint and deactivate every last pig gene that contributes to organ rejection. “Maybe not in my lifetime, but we will get to a stage where we can modify the pig to an extent that we will not have to even use immunosuppressive drugs,” Mohiuddin says.
This would also eliminate the second major risk—infection—since immunosuppression increases the risk of infection in all transplants. “In human-to-human transplant, we see lots of infection because of the immunocompromised state of the recipient, and we have learned to overcome those infections. We hope that in xenotransplantation, it’ll be the same,” Mohiuddin says. However, it’s no guarantee that these techniques will work in xenotransplants, and they may even trigger rejection.
The third challenge, unique to xenotransplants, is preventing transmission of pig diseases to human recipients, which could then spread to the health care team, friends and family. The U.S. Food and Drug Administration (FDA) requires strict protocols for raising and testing donor pigs and monitoring recipients and their contacts post-transplant. The worry includes diseases that could be lying dormant in the pig DNA, so some pigs are genetically edited to inactivate these genes.
In cases so far, disease transmission hasn’t been an issue, and Mohiuddin doesn’t think it will become one. “But we don’t know what we don’t know, so that’s why we need to follow [patients],” he says.
Pig Hearts to Humans
In 2022, a team at the University of Maryland Medical Center performed the first ever gene-edited organ xenotransplant—of a heart—into a living human. The FDA granted compassionate use clearance to transplant a pig heart into David Bennett, a 57-year-old man with terminal heart disease. He had been admitted to the hospital, was bed-ridden for two months and was not eligible for a typical transplant.
“We were not sure whether, after putting in this pig heart, this patient would even wake up,” says Mohiuddin, a member of Bennett’s xenotransplant team.
The transplant went well, and Bennett did not suffer acute rejection. He lived for 60 days post-transplant. For nearly 50 of them, he was healthy and even began regaining strength in physiotherapy, Mohiuddin says. But after a short decline, Bennett died from heart failure. His care team found evidence of a dormant pig virus in his system, raising questions about whether it could have contributed to the transplant failure. However, they found no proof of this, and that virus isn’t able to infect human cells.
Another potential contributor: Bennett’s doctors twice administered IVIG antibodies to him to help fight infection and rejection—typical protocol in transplant patients. However, they realized later that the antibody mixture contained anti-pig antibodies that may have aggravated Bennett’s heart.
Mohiuddin’s team was hopeful that the second heart xenotransplant would be a greater success because Lawrence Faucette, age 58, despite having terminal heart disease, was healthy enough to walk when they began applying for compassionate use clearance from the FDA. Unfortunately, Faucette declined quickly, and his original heart arrested twice before the transplant. He suffered post-surgical complications and blood loss, and the blood transfusion he received contained high levels of anti-pig antibodies, as his doctor later discovered, which worsened his condition. Additionally, Faucette’s xeno heart couldn’t reach a heart rate that would perfuse his whole body, so his care team had to artificially increase it, which may have damaged it further. He died from organ rejection 40 days later.
Mohiuddin’s team is now seeking a healthier patient to see how a xeno heart functions when the original heart is the only damaged organ a person has. But volunteers must have no other options to qualify for compassionate use, so they must be really sick. “We will never get a perfect patient because a perfect patient will first opt in for a human heart,” Mohiuddin says.
United Therapeutics is finishing a baboon study that should provide the data necessary to apply for a xeno heart clinical trial, which the company hopes to complete within the year. If approved, a clinical trial would allow for dozens of more people to receive a xeno heart, giving researchers the opportunity to tweak the process.
For now, xeno heart researchers must look to the first clinical trial in kidneys for steps to improve survival and fight rejection.
Pig Kidneys to Humans
Compassionate use cases are critical for learning how to make xenotransplants successful, but scientists can’t use them alone as evidence to convince the FDA of their safety or efficacy—or to convince the agency to approve a clinical trial.
For this, the FDA requires controlled studies of the genetically engineered organ in baboons that mimic a proposed clinical trial, to the extent possible. Earlier studies were conducted using pig organs with various gene edits, different immunosuppression regimens or other uncontrolled factors.
“There was a lot of mixing and matching to determine the best combination. That’s the nature of how science is done at that stage,” says Leigh Peterson, PhD, executive vice president of product development and xenotransplantation at United Therapeutics.
In a lead-up to applying for a clinical trial, United Therapeutics consulted with the FDA and ran a comprehensive 14-baboon kidney xenotransplant experiment—to successful results. After the company submitted a 1,200-page application on New Year’s Eve in 2024, the FDA granted it clearance for the first clinical trial of a gene-edited pig organ transplant to people. Around the same time, the FDA also cleared Massachusetts General Hospital to begin a small study using pigs with more gene edits, provided by another biotech company called eGenesis.
The United Therapeutics trial will begin with two transplants, each followed by a 12-week waiting period to analyze safety and efficacy. Then four more patients will receive a xeno kidney, followed by another 12-week waiting period. If the results are encouraging, the trial will continue until up to 50 xenotransplants are conducted. Along the way, United Therapeutics may amend the protocol based on what its researchers learn to reduce chances of rejection and reverse it if it occurs. “We are learning more and more how to identify and treat xeno organ rejection episodes,” Peterson says.
Part of why Peterson is so confident is that compassionate use cases have met success. Of the five people who have received xeno kidneys through compassionate use, three are still alive: 53-year-old Towana Looney, 66-year-old Tim Andrews and a 69-year-old woman in China about whom less is known. Looney received her new kidney on November 25, 2024, after waiting seven years for a transplant. However, in March 2025, her body began to reject the organ for unknown reasons, and she had it removed on April 4 to go back on dialysis. Andrews received his pig kidney on January 25 this year and is still healthy and free from dialysis.
Xeno kidneys appear to function well, especially in filtration and urine production. But there could be some physiological challenges, says Kelly Hyndman, PhD, a medicine professor specializing in nephrology at the University of Alabama at Birmingham School of Medicine. For example, kidneys are important for regulating blood pressure, and it’s unclear how xeno kidneys will fare doing this in the long term. If blood pressure rises, clinicians will need to test whether common medications are safe and effective for xenotransplant patients.
Additionally, kidneys must produce and respond to various hormones, and it’s unclear whether the xeno organs will be able to do so effectively. Preliminary research shows that the hormone renin produced by the pig kidney may not be able to interact correctly with the hormone angiotensin produced by the human liver—which is crucial for maintaining sodium and water balance and regulating blood pressure, Hyndman says.
Furthermore, it’s unknown how well the xeno kidney-produced hormone erythropoietin will be able to perform its function of spurring bone marrow to make red blood cells, crucial for preventing anemia.
However, these potential complications are easily monitored, Hyndman says, and can be treated by giving the patients exogenous hormones. Nephrologists are already well-versed in doing so. “Dialysis does not help with any of those things; all dialysis does is remove things from your blood,” Hyndman says.
No More Waitlists
If clinical trials succeed and xenotransplants become available to the public—a future that might be just a few years away for the kidney and heart—innumerable lives will be saved. “We want to create an unlimited supply [of organs] and give patients another option other than, in some cases, death, and in some cases, a lifetime of dialysis,” Peterson says.
It’s not only patients with heart and kidney failure who could benefit from xenotransplants. Researchers are studying xenotransplantation of the liver and lungs, too. In March, Chinese researchers reported the first xeno liver transplant to a brain-dead recipient. Instead of removing the human liver, they placed the pig liver beside it, and it survived for the 10 days of the experiment with no signs of rejection. The xeno liver produced bile and albumin, though less than a human organ would.
There’s still a long way to go before scientists xenotransplant a liver into a living human because the organ has many additional functions—from removing waste to fighting infection to storing iron and more—and it’s unclear how well a xeno liver would perform these functions. But even if the xeno liver survives only a few days, it could save patients’ lives. “When patients develop liver failure, they sometimes need an organ right away,” Riella says. “To even have a liver working for a few days, until the patient either recovers or receives an offer from a human,” could be the bridge that keeps them alive, he says.
Researchers are also studying xeno lungs, although they haven’t yet moved from the animal model stage to humans, and the complex anatomy of the lung poses challenges. Eventually, other organs could be xenotransplanted as well, such as pig skin for burn victims and pancreatic islets for people with diabetes.
Researchers are already considering the ethical concerns of scaling up xenotransplants for widespread use. For instance, United Therapeutics is trying to limit the environmental impact of raising pigs with plans to use one pig for multiple organs. The company is also anticipating ethical concerns from the public. However, the ethical issue is another reason pigs are good donors: About 1.4 billion pigs are slaughtered every year for meat, which for many people is not necessary for survival, whereas pigs killed for xenotransplantation will directly save lives. Mohiuddin says even leaders of religions that don’t allow pork consumption have said they would allow practitioners to accept a xenotransplant to save their life.
And many, many lives it could save. “If we can get this right, this is really going to help a lot of people,” Hyndman says.
This article was originally published in the July 2025 issue of The Physiologist Magazine. Copyright © 2025 by the American Physiological Society. Send questions or comments to tphysmag@physiology.org.
The Physiologist Magazine
Read the Latest Issue
Don’t miss out on the latest topics in science and research.
Contact Us
For questions, comments or to share your story ideas, email us or call 301.634.7314.


